Molecular mechanisms of Helicobacter-induced gastric cancer
Background
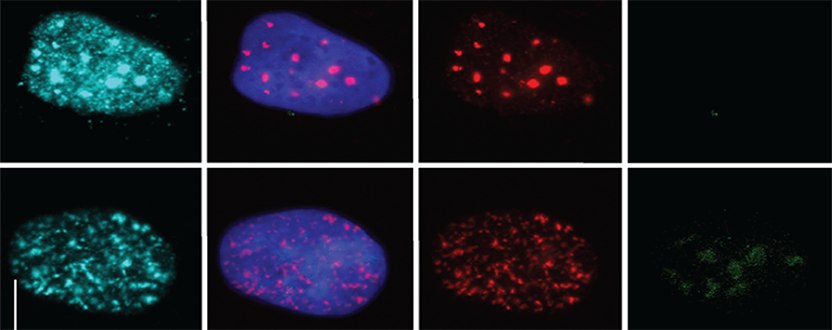
Gastric cancer is a common and extremely aggressive malignancy. Chronic infection with the gastric bacterial pathogen Helicobacter pylori has been identified as a main risk factor for this disease; H. pylori strains that express a functional type IV secretion system (T4SS) are much more tightly linked to gastric carcinogenesis than T4SS-negative strains. Our working model is that several events need to coincide to create a scenario in which malignant transformation can occur. On the one hand, an excessive, T-helper-1 (Th1)-driven immune response to the bacteria favors the development of chronic atrophic gastritis accompanied by compensatory epithelial hyperplasia, a well-known gastric cancer precursor lesion. Hyperproliferating (hyperplastic) epithelial cells in turn become susceptible to replication stress; recent data from our lab implicate a newly described T4SS/b-ADP-heptose/ALPK1/TIFA signaling axis, leading to NF-kB activation and active transcription of NF-kB target genes, in replication stress specifically in such actively proliferating (S-phase) cells. NF-kB-driven transcription occurring in S-phase cells results in the formation of RNA/DNA hybrids (so-called R-loops) at sites where transcription and replication machineries collide. R-loop structures that are not properly resolved promote DNA damage as they are recognized by endonucleases of the nucleotide excision repair machinery.
Goal
The main objective of this project is to unravel whether, and how Helicobacter pylori infection synergizes with mutations occurring early on in the preneoplastic cascade to drive DNA damage, the accumulation of additional mutations, genomic instability and gastric carcinogenesis.
Ongoing and future work
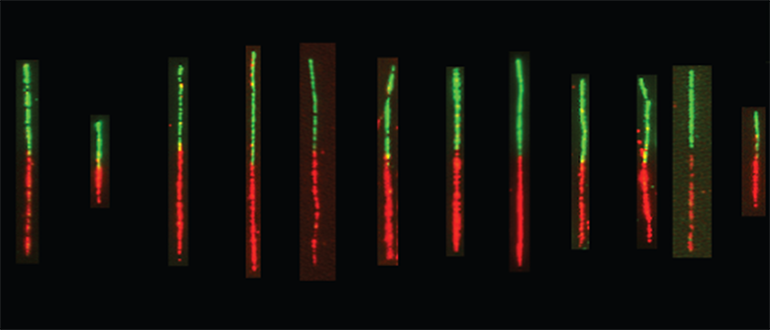
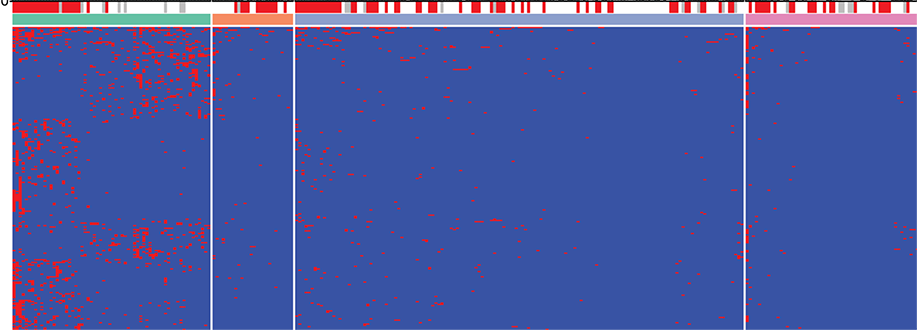
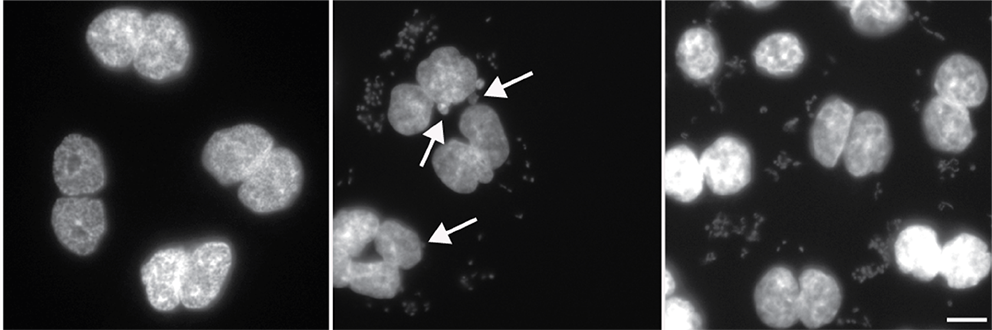
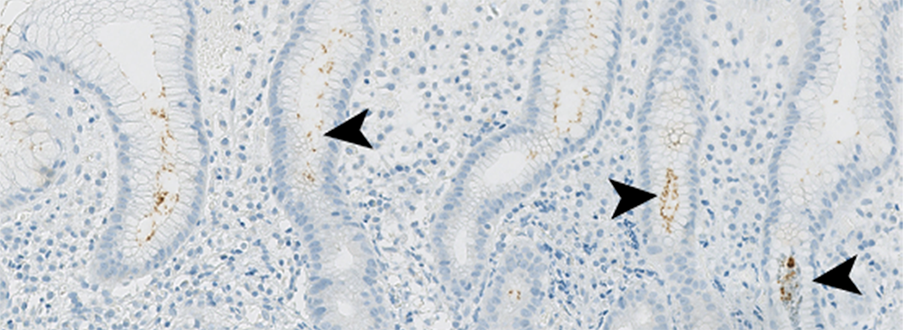
We are currently using cell culture systems and murine organoids, which we manipulate by RNAi and CRISPR, in conjunction with quantitative assays of DNA damage (pulsed-field gel electrophoresis, immunofluorescence microscopy, comet assay), replication stress (DNA fiber assay), genomic instability (micronuclei formation) and malignant transformation to elucidate how gastric cancer driver mutations and Helicobacter pylori infection act in concert to promote gastric carcinogenesis. We are using chromatin immunoprecipitation to identify sites of DNA damage in H. pylori target cells, in combination with bioinformatic analyses of publicly available gastric cancer patient data and suitable mouse models of gastric H. pylori-driven preneoplasia to investigate how infection-induced DNA damage predisposes to gastric carcinogenesis.
Selected publications
Bauer M, Nascakova Z, Mihai A, Cheng, P. Levesque, M.P., Lampart, S., Hurwitz R, Pfannkuch, L., Dobrovolna, J. Jacobs M, Bartfeld S, Dohlman, A., Shen, X., Gall T, Salama, N R, Töpfer, A., Weber, A., Meyer, T.F., Janscak, P.* and Müller A.* The ALPK1/TIFA/NF-kB axis links a bacterial carcinogen to replication stress and DNA damage, Nat. Commun. (2020)
Hartung, M.L., Gruber, D.C., Koch, K.N., Grüter, L., Rehrauer, H., Tegtmeyer, N., Backert, S. and Müller, A. Helicobacter pylori-induced DNA double strand breaks are introduced by nucleotide excision repair endonucleases and promote NF-kB target gene expression. Cell Reports. 13:70-9 (2015).
Toller. I.M., Neelsen, K., Steger, M., Hottiger, M.O., Stucki, M., Gerhard, M., Sartori, A.A., Lopes, M. and Müller, A. The carcinogenic bacterial pathogen Helicobacter pylori triggers DNA double strand breaks and a DNA damage response in infected host cells. PNAS 108, 14944-14949 (2011).