Development and function of regulatory T-cells in settings of chronic bacterial infection and cancer
Background
Regulatory T-cells (Tregs) represent a subset of CD4+ T-cells that express Foxp3 and play a central role in immune tolerance to self and foreign antigens. Self/autoreactive Foxp3+ Tregs are generated during thymic T cell development. In addition, Tregs with specificities for food antigens or microbiota, or for infectious agents, arise by peripheral conversion of conventional Foxp3−CD4+ T cells into Foxp3+ Tregs; such “peripherally induced” (p)Tregs can develop at various sites in the body but are particularly abundant in gastro-intestinal tissues and their draining lymph nodes. During infection, pTregs function to suppress excessive pathogen-specific effector T-cell responses and the resulting immunopathological tissue damage, and restore tissue homeostasis upon pathogen clearance. We study pTreg induction in settings of acute and chronic infection with bacterial pathogens, and as a consequence of the intergenerational acquisition of a diverse microbiota in the first weeks of life. We are particularly interested in understanding the contribution of various populations of myeloid cells (especially dendritic cells) to peripheral Treg induction, in the microbial signals governing Treg conversion from naïve T-cells and in the consequences of pTreg dysfunction for the development of allergies, autoimmune diseases and other immunopathologies.
Goal
The main objectives of this project are to gain a better understanding of the myeloid cells and granulocyte lineages involved in Treg induction in the periphery, the microbial drivers of Treg conversion acting on myeloid cells and directly on T-cells to promote Treg priming, and the consequences of defective pTreg responses in settings of bacterial infection, allergic responses and cancer.
Ongoing work
We are using mouse models of four bacterial pathogens (Helicobacter pylori, Helicobacter hepaticus, Mycobacterium bovis BCG and Citrobacter rodentium), which are complementary in terms of their pathogenesis, site of infection, chronicity and the type of T-cell responses they induce, alongside various cancer models to study the cellular and molecular prerequisites of Treg development and the function of Treg subsets in suppressing immunopathology and tumor control at various sites. We use conditional ablation of key signaling pathways in the Treg and in myeloid cell compartments in combination with the above listed models, and in models of antibiotic-induced dysbiosis to elucidate pTreg priming, migration, expansion and function.
Selected publications
Arnold, I.C., Artola-Boran, M., Gurtner, A., Bertram, K., Bauer, M., Frangez, Z., Becher, B., Kopf, M., Yousefi, S. Simon, H., Tzankov, A. and Müller, A. The GM-CSF/IRF5 signaling axis in eosinophils promotes anti-tumor immunity through activation of type I T-cell responses. J Experimental Medicine, 217(12):e20190706 (2020).
Zhang, X., Artola-Boran, M., Fallegger,A., Arnold, I.C., Weber, A., Reuter, S., Taube, C. and Müller, A. IRF4 expression is required for the immunoregulatory activity of cDC2s in settings of chronic bacterial infection and cancer. J Immunology, 205:1933-1943 (2020).
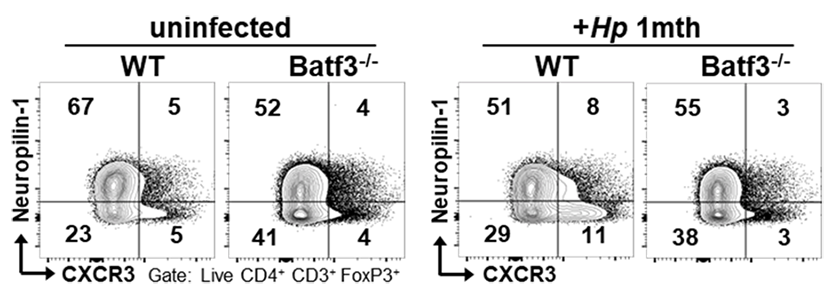
Arnold, I.C., Zhang, X., Artola-Boran, M., Fallegger, A., Sander, P., Johansen, P., Müller A. BATF3-dependent dendritic cells drive both effector and regulatory T-cell responses in bacterially infected tissues. PLoS Pathog. 15:6. (2019).
Altobelli, A., Bauer, M., Velez, K., Cover, T.L., Müller, A. Helicobacter pylori VacA Targets Myeloid Cells in the Gastric Lamina Propria To Promote Peripherally Induced Regulatory T-Cell Differentiation and Persistent Infection. MBio. 10:2 (2019).
Kyburz, A., Fallegger, A., Zhang, X., Altobelli, A., Artola-Boran, M., Borbet, T., Urban, S., Paul, P., Münz, C., Floess, S., Huehn, J., Cover, T.L., Blaser, M.J., Taube, C., and Müller A. Transmaternal Helicobacter pylori exposure reduces allergic airway inflammation in offspring through regulatory T cells. J Allergy Clin Immunol. 143:1496-1512.e11 (2019).
Arnold, I.C., Artola-Borán, M., Tallón de Lara, P., Kyburz, A., Taube, C., Ottemann, K., van den Broek, M., Yousefi, S., Simon, H.U., and Müller, A. Eosinophils suppress Th1 responses and restrict bacterially induced gastrointestinal inflammation. J Exp Med. 215:2055-2072 (2018).
Arnold, I.C., Urban, S., Zhang, X., Becher, B., Manz, M.G., Ottemann, K. and Müller, A. NLRP3 controls CD11b+ dendritic cell differentiation at steady state and during bacterial infection. Cell Reports 21:3860-3872 (2017).
Kyburz, K., Urban, S., Djekic, A., Floess, S., Huehn, J. Cover, T.L., and Müller, A. Helicobacter pylori and its secreted immunomodulator VacA protect against anaphylaxis in experimental models of food allergy. Clinical and Experimental Allergy. 47(10):1331-1341 (2017).
Engler, D.B., Reuter, S., van Wijck, Y., Urban, S., Kyburz, A., Maxeiner, J., Martin, H., Yogev, N., Waisman, A., Gerhard, M., Cover, T.L., Taube, C., and Müller, A. Effective treatment of allergic airway inflammation by tolerization with Helicobacter pylori-derived immunomodulators requires BATF3-dependent dendritic cell lineages and IL-10. PNAS, 111:11810-5 (2014).